Download the free whitepaper: Unravelling the complexities of genomics-driven drug discovery
Genomics-driven drug discovery holds immense promise in developing targeted therapies by leveraging genetic and biomarker information to understand the root causes of diseases. However, the field faces significant hurdles.
In this whitepaper, we examine the obstacles of limited recontactability, scarcity of multi-omics data sets, lack of diversity in data, and the challenge of scaling research for rare diseases. By addressing these challenges and proposing strategies to address them, this whitepaper aims to chart a path by which we can harness the full potential of genomics-driven drug discovery.

Download whitepaper
Conversion vs. compliance: Balancing patient centricity with regulatory restrictions
As the number of pioneering precision medicine clinical trials continues to expand, effectively balancing patient enrollment rates with stringent regulatory restrictions can seem increasingly challenging. In this whitepaper, “Conversion vs. compliance: Balancing patient centricity with regulatory restrictions,” we explore why compliance and conversion don’t need to be in tension and how building a patient-centric approach to trial recruitment leads to improved data quality, and streamlined, highly compliant workflows.
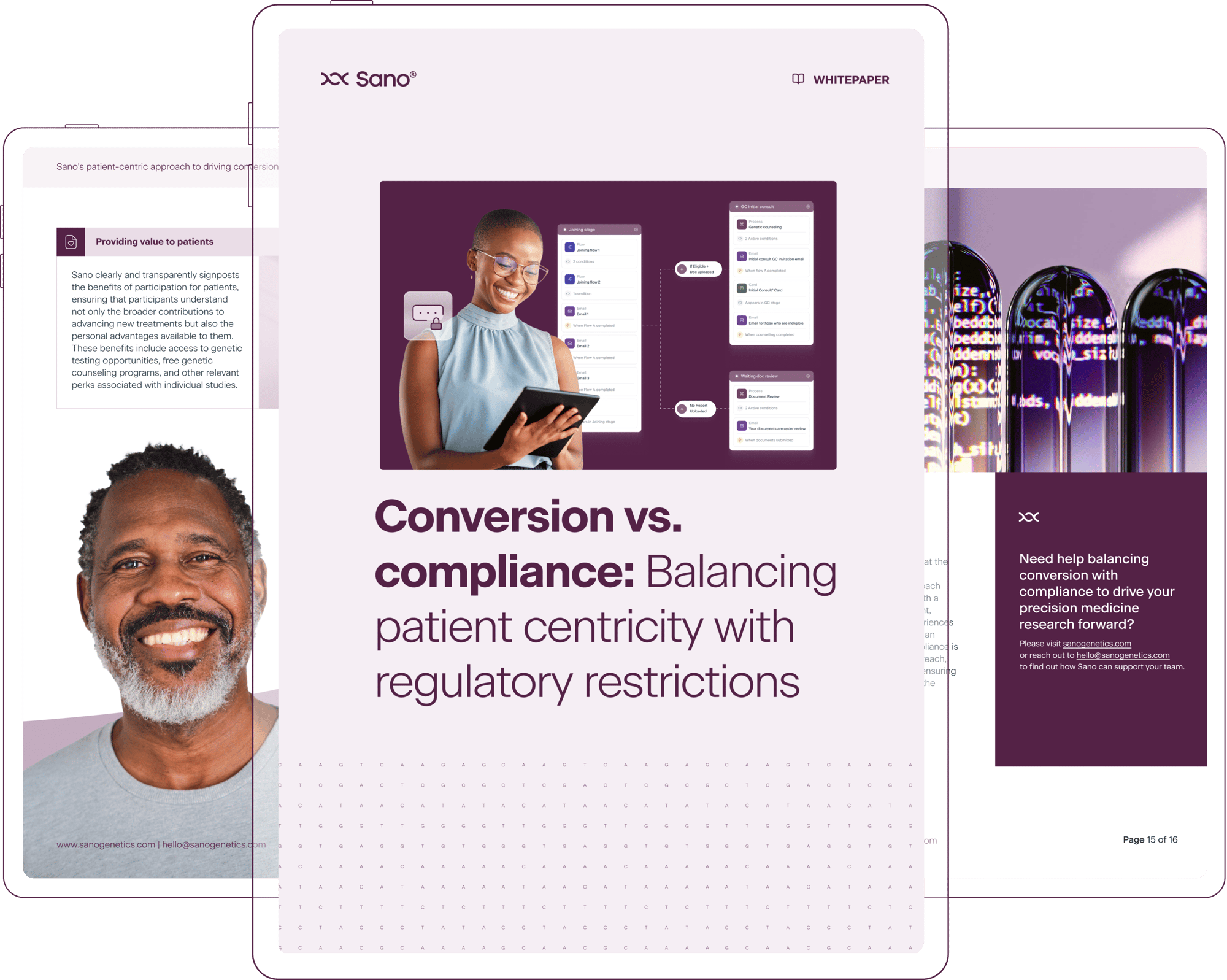
What’s inside:
🔄 Conversion: Going beyond regulatory box ticking to build user experience-led recruitment campaigns
✅ Compliance: How regulatory requirements aid development of long-lasting patient partnerships and enable access to high quality research data
⚖️ Balancing research goals with compliance: Overcoming points of tension, setting thresholds and enabling dynamic responses (and what this looks like at Sano).
Download to learn more.
